When BCR-ABL1 kinase domain
mutation analysis is recommended?
• Chronic phase
Failure to reach response milestones
Any sign of loss of response (defined as hematologic or cytogenetic relapse)
1-log increase in BCR-ABL1 transcript levels and loss of MMR
• Disease progression to accelerated or blast phase
BCR-ABL1 mutations that should NOT be treated with bosutinib, dasatinib or nilotinib in the second-line setting?
Bosutinib : T315I, V299L, G250E or F317Lp
Dasatinib T315I/A, F317L/V/I/C or V299L
Nilotinib T315I, Y253H, E255K/V, or F359V/C/I or G250E
Ponatinib, Omacetaxine, allogeneic HCT ,or clinical trial : None

What are definitions for different kind of responses?
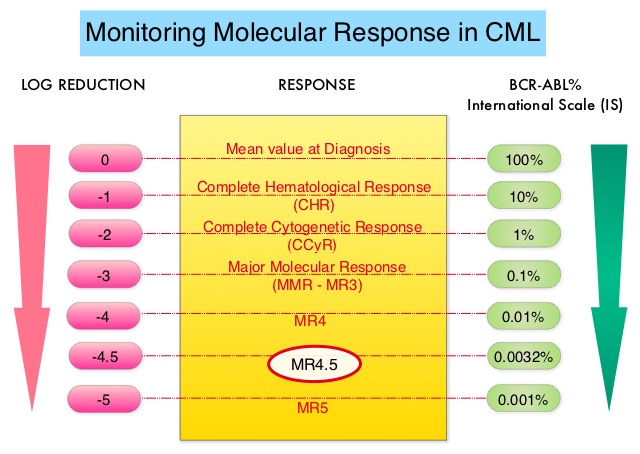
Molecular response:
• Early molecular response (EMR) - BCR-ABL1 (IS) ≤10% at 3 and 6 months
• Major molecular response (MMR) - BCR-ABL1 (IS) ≤0.1% or ≥3-log reduction in BCR-ABL1 mRNA from
the standardized baseline, if qPCR (IS) is not available
• Complete molecular response (CMR) is variably described, and is best defined by the assay’s level of
sensitivity (eg, MR4.5)
Relapse:
• Any sign of loss of response (defined as hematologic or cytogenetic relapse)
• 1-log increase in BCR-ABL1 transcript levels with loss of MMR should prompt bone marrow evaluation
for loss of CCyR but is not itself defined as relapse (eg, hematologic or cytogenetic relapse)
What are NCCN and ESMO recommendations for treatment discontinuation ?


When BCR- ABL is undetectable which factors define sensitivity and reliability of the test?

According to good laboratory practice and guidelines, what are minimum requirements for BCR-ABL test reports?

- The goal of TKI therapy is to achieve a CCyR (≤1% BCR-ABL1 IS) within 12 months after first-line TKI therapy and to prevent disease progression to AP-CML or BP-CML. The guidelines emphasize that achievement of response milestones must be interpreted within the clinical context, before making drastic changes to the treatment strategy.
- The NCCN panel has included ≤10% BCR-ABL1 IS at 3 and 6 months and ≤1% BCR-ABL1 IS at 12 and 15 months as response milestones after first-line TKI therapy. Patients who achieve these response milestones are considered to have TKI-sensitive disease, and continuation of the same dose of TKI and assessment of BCR-ABL1 transcripts with qPCR (IS) every 3 months is recommended for this group of patients.
- In patients with a >10% BCR-ABL1 IS at 3 months and >1% BCR-ABL1 IS at 12 months, clinical judgment should be used, considering problems with adherence (which can be common given drug toxicity at initiation of therapy), rate of decline in BCR-ABL1 (the faster, the better), and how far from the cutoff the BCR-ABL1 value falls. That being said, failure to achieve ≤10% BCR-ABL1 IS at 3 months or ≤1% BCR-ABL1 IS at 12 months is associated with a higher risk for disease progression. Patients with >10% BCR-ABL1 at 3 months or >1% BCR-ABL1 at 12 months can continue the same dose of dasatinib or nilotinib or bosutinib for another 3 months. BCR-ABL1 mutational analysis and evaluation for allogeneic HCT should be considered. Bone marrow cytogenetics should be considered to assess for major cytogenetic response (MCyR) at 3 months or CCyR at 12 months.
- Patients with >10% BCR-ABL1 IS at ≥6 months and those with >1% BCR-ABL1 IS at 15 months are considered to have TKI-resistant disease. Evaluation for allogeneic HCT (that is, a discussion with a transplant specialist, which might include HLA testing) is recommended. Alternate treatment options should be considered as described below.

Resistance to TKI Therapy
-
Aberrant expressions of drug transporters and plasma protein binding of TKI could contribute to primary resistance by altering the intracellular and plasma concentration of TKI.
- Pretreatment levels of organic cation transporter 1 (OCT1) have been reported as the most powerful predictor of response to imatinib. On the other hand, cellular uptake of dasatinib or nilotinib seems to be independent of OCT1 expression, suggesting that patients with low OCT1 expression might have better outcomes with dasatinib or nilotinib than with imatinib.
- Monitoring imatinib plasma levels may be useful in determining patient adherence to therapy. However, there are no data to support that change of therapy based on plasma imatinib levels will affect treatment outcomes.